- Aims and Scope
- Article Types
- Manuscript Preparation
- Clinical Trials
- Online Manuscript Submission
- Peer Review Process
- Editorial Policy and Publication Ethics
- Ethical Policies and Handling of Misconduct
- Proofing and Revision after Acceptance
- Article Processing Charge (APC)
- Copyright
Spine Surgery and Related Research
Aims and Scope
Spine Surgery and Related Research (SSRR), an official open-access journal of the Japanese Society for Spine Surgery and Related Research (JSSR), is an international, peer-reviewed, multidisciplinary journal directed to spine physicians and scientists. SSRR publishes original articles in the form of basic, clinical, and translational research on anatomy, pathophysiology, biomechanics, diagnosis, medical and surgical treatments related to spine and spinal cord. SSRR accepts review articles from evidence-based perspective including systematic review, suggestive technical notes, and rare clinical cases. The Journal is published bi-monthly (January, March, May, July, September and November). SSRR requires that all manuscripts be prepared in accordance with the ICMJE Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly work in Medical Journals.
Article Types
SSRR publishes the following article types. Once you have determined the correct Article Type, it is imperative that you read and follow the descriptions provided in the Manuscript Preparation guidelines before you submit your manuscript:
a) Review Articles
Review Articles should provide a broad overview and updates on a specific field within the scope of SSRR.
b) Original Articles
Original Articles should present detailed studies of original research highlighting new and compelling findings that are impactful to other medical practitioners and researchers in the spine surgery field.
c) Technical Notes
Technical Notes present the tips pertaining to surgical techniques for traditional/novel surgical procedures with their clinical outcomes.
d) Clinical Correspondence
Clinical Correspondence presents the details of rare medical or clinical cases. Clinical Correspondence papers will not be accepted unless they are extremely instructive/informative. They may be suggested for transfer to another journal, including Journal of Spine Research (JSR), an official domestic journal of the JSSR, based on editorial decision that they are not a good fit for SSRR.
e) Letter to the Editor
Letters to the Editor are brief, constructive commentaries that can be submitted in response to a recently published article in SSRR.
Manuscript Preparation
The information provided below is based in part on “Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly work in Medical Journals,” as published by the International Committee of Medical Journal Editors (ICMJE). For any information that is not mentioned in these guidelines, authors should refer to the ICMJE Recommendations.
Manuscripts that do not follow the instructions below WILL BE RETURNED to the corresponding author for technical revision before undergoing further review.
General Formatting
All articles should be written in English and formatted as per the standard letter size [8 1/2 × 11 inch (21 × 28 cm)] paper with at least 1-inch (2.5 cm) margins on all sides. All elements of the manuscript, including Abstract, Main Text, References, Tables, and Figure Legends, should be typed double spaced. Line numbers and page numbers on each page are required to make it easier for reviewers to provide their comments.
The organization of the manuscript should be in the following order:
- Title page
- Abstract
- Key Words
- Main Text
- References
- Tables
- Figure Legends
- Figures
- Supplemental Files (e.g., videos, if applicable)
Title Page
The title page should be prepared and saved in a separate file from the main document to ensure anonymity of the manuscript during the review process.
The title page must include the following information:
- Title of the manuscript
- Names of all authors
- Institutional affiliations of all authors
- Corresponding author’s name, address, telephone number and e-mail address
- Conflicts of Interest for all authors
- Sources of financial support that require acknowledgment
- Contributions to the submitted work from each author. Please visit the ICMJE website for more information on authorship
- Acknowledgements (if applicable)
- Approval code issued by the institutional review board (IRB) and the name of the institution(s) that granted the approval. If no approval from any IRB was required, the reason(s) must be stated explicitly.
- A statement that appropriate informed consent was obtained. If the consent from the participants was waived for your study, the reason(s) must be stated explicitly.
*SSRR Title Page Template can be downloaded.
Abstract and Key Words
The manuscript should include a structured Abstract of no more than 300 words and must contain the following headings (as per the Article Type):
Review Articles:
– Narrative Review: Unstructured or structured Abstract
– Systematic Review: Structured Abstract (Background, Methods, Results, Conclusions)
Original Articles: Structured Abstract (Introduction, Methods, Results, Conclusions)
Technical Notes: Introduction, Technical Note, Conclusions
Clinical Correspondence: None
Abstracts, regardless of the Article Type, should contain a list of three to eight key words. Reports of clinical trials must include the registration number and name of the registration database in the abstract. See further information on clinical trials below.
Main Text
For each Article Type, authors must organize and order their content using the following formats:
Review Articles
- Main Headings:
– Narrative Review: Not required
– Systematic Review: Introduction, Materials and Methods, Results, Discussion - Word limit: 4,000 words
- Number of Tables: No more than 7
- Number of Figures: No more than 10
Original Articles
- Main headings: Introduction, Materials and Methods, Results, Discussion
- Word limit: 2,700 words
- Number of Tables: No more than 5
- Number of Figures: No more than 6
Technical Notes
- Main headings: Introduction, Technical Note, Discussion
- Word limit: 1,500 words
- Number of Tables: No more than 3
- Number of Figures: No more than 8
Clinical Correspondence
- Word limit: 600 words
- References: No more than 10
- Number of Tables and Figures: No more than 4
Letter to the Editor
- Word limit: 400 words
- Number of Tables and Figures: No more than 2
Specifications by Article Types
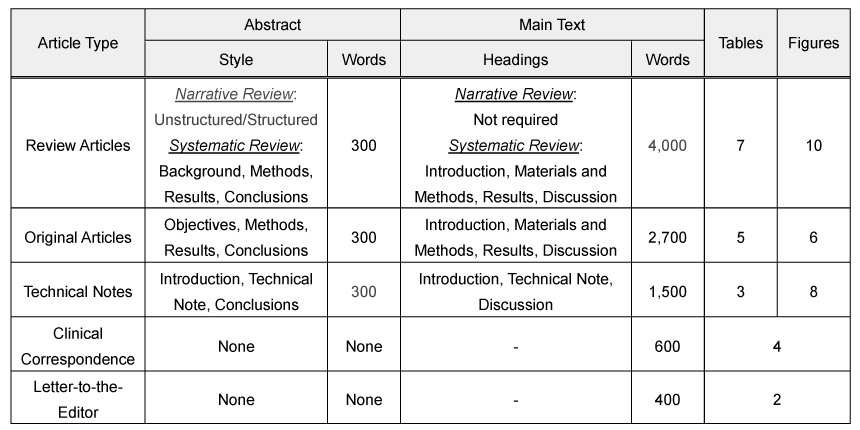 The main text does not include title page, abstract, references, figure legends and table titles.
The main text does not include title page, abstract, references, figure legends and table titles.
References
The authors are responsible for the accuracy of their references.
- List the references immediately after the main text.
- The references should be numbered in the order of their appearance in the main text. Do not list the references in alphabetical order.
- References should be indicated in the main text by using superscripts (for example, show in superscript1) ).
- Including AI-generated material as the primary source in the reference is not allowed.
- If there are more than three authors, name only the first three authors and then use “et al.”
- Journal names should be abbreviated in the standard form as they appear in the NLM catalog. If the journals are not included in the NLM catalog, use the ISSN List of Title Word Abbreviations for standard abbreviations of journal names. If you are uncertain, please use the full journal name.
For reference styles pertaining to other media formats or further details, please refer to Citing Medicine, which is published by the National Library of Medicine (US).
Reference examples follow:
Journal article
1. Guiot BH, Khoo LT, Fessler RG. A minimally invasive technique for decompression of the lumber spine. Spine. 2002;27(4): 432-8.
Journal article in a language other than English
2. Paroussis D, Papaoutsopoulou C. [Porcelain laminate veneers (HI-ERAM)]. Odontostomatol Proodos. 1990;44(6):423-6. Greek.
Online journals with DOI
3. Harrison JJ, Ceri H, Yerly J, et al. The use of microscopy and three-dimensional visualization to evaluate the structure of microbial biofilms cultivated in the Calgary Biofilm Device. Biol Proc Online. 2006;8(1): 194-215.doi:10.1251/bpo127
Entire book
4. Jenkins PF. Making sense of the chest x-ray: a hands-on guide. New York: Oxford University Press; 2005. 194 p
Book chapter
5. Riffenburgh RH. Statistics in medicine. 2nd ed. Amsterdam (Netherlands): Elsevier Academic Press; c2006. Chapter 24, Regression and correlation methods; p. 447-86.
Software
6. Mayo Foundation for Medical Education and Research. The total heart: the ultimate interactive guide to heart health [CD-ROM]. PC 1.1a version. Eagan (MN): IVI Pub.; 1993. 1 CD-ROM: sound, color, 4 3/4 in. Accompanied by: 1 manual.
Database/Internet Document
7. MeSH Database [Internet]. Bethesda (MD): National Library of Medicine (US). 2003 Apr – [cited 2011 Jul 8]. Available from: http://www.ncbi.nlm.nih.gov/mesh
For authors using EndNote, you can use the output style below for citations and reference list. SSRR_EndNote_Style.zip (powered by USACO cooperation)
Abbreviations
Define abbreviations at their first appearance in the abstract, in the main body of text, and in tables and figure legends, and use the abbreviations consistently thereafter. Do not include abbreviations in the title.
Names of Drugs, Devices, and Other Products
Do not use the specific brand names of drugs, devices, and other products and services, unless it is essential to the discussion. Otherwise, please use descriptive name only.
Unit of Measurement
All measurements should be in the metric system and follow the International System of Units (SI).
Temperatures should be in degrees Celsius. Blood pressures should be in millimeters of mercury. All measurements should follow the International System of Units (SI).
Use a capital letter “L” for liter in the units of measurements in the Text, Figures, and Tables (e.g., g/dL, mg/dL, IU/L, and mEq/L).
Tables and Figures
All Tables and Figures should be submitted in the following digital format: MS Word (.doc/.docx), JPEG (.jpg), or Tagged Image Format (.tiff). Do not use MS Excel or comparable spreadsheet software. Figures and Tables must be cited in the text and numbered in the order they are cited.
Tables
- All tables are required to be in MS Word (.doc/.docx) or PowerPoint (.ppt/.pptx) as editable files, not pasted as images.
- Include a brief title for each table.
- Put all explanatory matter in footnotes, including an explanation for all nonstandard abbreviations used in table.
Figures
- Images should be at the minimum resolution of 300 dpi. Include the scale (bar) in images captured with scanning electron microscopes.
- Figures supplied within the main manuscript Word document or previously copy and pasted into PowerPoint are not acceptable. This is due to their low resolution. They will not re-produce in print or online clearly.
- Scanned images of line art will not be accepted – please supply in the original file format.
- Tone art, or photographic images should be produced at the minimum resolution of 300 dpi. Include the scale (bar) in images captured with scanning electron microscopes.
- All figure titles and legends should not be embedded in the submitted image – please supply this information separately (such as figure legends in the main manuscript file).
- All extraneous use of color must be removed from figures and tables. Color should only be used for didactic purposes. All line art backgrounds must not contain any color.
Figure Legends
Legends must be prepared for all Figures presented in the manuscript. Authors must list Figure Legends on a separate page after the references.
If any copyrighted or previously published material, edited or otherwise, are used in the manuscript, it is the author’s responsibility to obtain permission from the copyright owner(s) prior to submitting the manuscript. Also, the authors must cite the source and indicate the permission to use such materials in the corresponding Figure or Table caption, as required by the copyright owner(s).
Supplementary Material
SSRR accepts supplementary materials that may contain additional figures, tables or supporting movies directly relevant to the article content. Supplementary materials are published exactly as they are received and not edited by the journal. The same policies for ethics, copyright, permissions and publication quality for the main article apply to the supplementary materials. Authors should submit the supplementary materials as “Supplemental Files” during the manuscript submission process via ScholarOne Manuscripts submission system. All video files should be submitted in common video file formats (e.g..mpg/.mov/.wmv/.mpeg/.mp4/.avi). Refer to the supplementary materials starting with S1, S2 (e.g. Fig. S1, Table S1, Video S1) in the main text. All supplementary materials will be published alongside the article on the journal website.
Clinical Trials
In accordance with ICMJE’s policy on trial registration, all clinical trials must be registered with a public trials registry before the time of first patient enrollment. ICMJE defines clinical trials as any research project that prospectively assigns people or a group of people to an intervention, with or without concurrent comparison or control groups, to study the cause-and-effect relationship between a health-related intervention and a health outcome. Health-related interventions include but are not limited to those used to modify a biomedical or health-related outcome; examples include drugs, surgical procedures, devices, behavioral treatments, educational programs, dietary interventions, quality improvement interventions, and process-of-care changes.
SSRR requires all clinical trials to be registered with databases that are accessible to the public at no charge, open to all prospective registrants, managed by a not-for-profit organization, have a mechanism to ensure the validity of the registration data, and are electronically searchable.
Submitted manuscripts must include the unique registration number in the abstract as evidence of registration. The name of the registration database must also be provided. For details regarding the required minimal registration data set, please go to the International Committee of Medical Journal Editors’ (ICMJE) website.
The journal accepts registration from the following list of registries as well as others listed at ICMJE website:
- Clinical Trials
- Australian New Zealand Clinical Trials Registry
- ISRCTN Register
- UMIN Clinical Trials Registry
- EudraCT
In reporting randomized clinical trials, authors must comply with published CONSORT guidelines (http://www.consort-statement.org/). The recommended checklist must be completed and provided to the Journal at the time of manuscript submission. The recommended trial flow diagram should be presented as a figure.
Reporting Guidelines
Various reporting guidelines have been developed for different study designs. Authors are encouraged to follow published standard reporting guidelines for the study discipline.
- CONSORT for randomized clinical trials – this guideline is required for all randomized clinical trials, as noted above.
- CARE for case reports
- STROBE for observational studies
- PRISMA for systematic reviews and meta-analyses
- STARD for studies of diagnostic accuracy
- SAGER for reporting of sex and gender information
Please access http://www.equator-network.org/ to find the guideline that is appropriate for your study.
It is extremely important that when you complete any Reporting Guideline checklist, you consider amending your manuscript to ensure your article addresses all relevant reporting criteria issues delineated in the relevant reporting checklist prior to submission. The purpose of a reporting guideline is to guide you in improving the reporting standard of your manuscript. The objective is not to solely complete the reporting checklist, but to use the checklist itself in the writing of your manuscript. Taking the time to ensure your manuscript meets these basic reporting needs will greatly improve your manuscript, while also potentially enhancing its chances for eventual publication.
Data Sharing
SSRR encourages the authors of manuscript which includes clinical trials to share their de-identified research data including, but not limited to raw data, processed data, software, algorithms, protocols, methods, materials, study protocol, statistical analysis plan, informed consent form, clinical study report, analytic code.
As required by ICMJE, all manuscripts that report the results of clinical trial must include a data sharing statement with a link to the trial registration. The statement should include the following information:
- Available types of data,
- Available documents (study protocol, statistical analysis plan, informed consent form, clinical study report, or analytic code)
- Available dates
- With whom the data are available.
- Types of analyses the authors are willing to share the data
- Method of requesting the data.
The statement is published alongside their paper.
Online Manuscript Submission
Submit manuscript files electronically via the ScholarOne system in the following order: Title page, Main Text, Tables, and Figures (≥300 dpi). The total size of the uploaded files should be within 100 MB. Upon submission, the manuscript will be automatically checked for plagiarism by the iThenticate plagiarism screening service to determine both textual overlap and manuscript originality. The submitted manuscript can be sent back to the corresponding author for rewriting if the detected text overlap rate is 30% or higher.
Peer Review Process
Peer review is a critically important process of evaluation for any manuscript submitted to SSRR. Every article dispatched for full peer review will receive a comprehensive, fair, unbiased critical assessment. SSRR employs a double-anonymized review process. This means the identities of the peer reviewers and the authors remain anonymous to each other.
The main document of the submitted manuscript should adhere to the following requirements:
- Not include the name of the affiliation anywhere in the manuscript, including the Figures and Tables.
- Refer to the authors’ previous work as that of a third person, e.g., replace “…as we have reported in our previous study19” with “as it has been reported previously19”
- Not include the references to funding sources, such as identifier of the government-related funds.
- Not include acknowledgments.
- Declare the Conflicts of Interest (COI) on the title page (if applicable).
All submitted manuscripts will be initially reviewed by an editor of SSRR to evaluate eligibility for publication. The editor will assess the importance and originality of the research, suitability and interest to the readership of the Journal, and the validity and quality of the manuscript. Any manuscripts that satisfy our screening criteria will generally be sent to two experts in the field of study for peer review. The editor will review the peer review comments and make a decision for acceptance or rejection, or request that the authors revise the manuscript based upon the reviewers’ comments.
SSRR adheres to Committee on Publication Ethics’ Ethical Guidelines for Peer Reviewers. Reviewers are not allowed to contact the authors directly before, during, or after the reviewing process to discuss any information that is presented in the manuscript. Reviewers must keep the manuscripts and information obtained strictly confidential and must not publicly discuss or disclose the contents and any other information of the manuscript to a third party. The guidelines for the reviewers are available here.
The editors of SSRR make all decisions on the manuscript publication, which include acceptance, major or minor revisions, and rejection. The decision letters, along with the comments of the editors and reviewers, will be sent to the corresponding author via e-mail.
Revised Manuscript
Revised manuscripts must be fully amended to address the comments of both the reviewers and the editors. Authors must include an anonymized, detailed point-by-point response to the reviewers’ and editors’ comments when submitting a revised manuscript. Authors should submit the revised manuscript within 3 months from the date of the prior decision. All authors must approve every revision, correction and amendment prior to re-submission of the revised manuscript.
Editors and Journal Staff as Authors
Manuscripts submitted by editors, editorial committee members, or journal staff will follow the same process as outlined above. However, they are excluded from any editorial decision process of their own manuscript and have neither access to that manuscript nor any information about the review process other than what is provided in the editor’s decision letter. The editorial office will assign the paper to an editor who is not an author on the paper nor has any conflict of interest with the authors. Additionally, ScholarOne, the journal’s online submission and peer review system is designed to anonymized a person in other roles (editor/reviewer) from any paper he/she has authored. The manuscript submitted by editors, editorial committee, and journal staff of SSRR should include a statement that declares their personal conflict of interest with the journal.
Editorial Policy and Publication Ethics
SSRR observes the highest standards in journal publication ethics. The journal supports and adheres to the industry guidelines and best practices, including Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals (http://www.icmje.org/recommendations/browse/) by the International Committee of Medical Journals Editors (ICMJE) and the Principles of Transparency and Best Practice in Scholarly Publishing (a joint statement by the Committee on Publication Ethics [COPE], the Directory of Open Access Journals [DOAJ], the World Association for Medical Editors [WAME] and the Open Access Scholarly Publishers Association [OASPA]; https://doaj.org/apply/transparency/).
Exclusive Submission
Articles that have been previously published or are being considered for publication in another journal in any language will not be accepted. The editors make all decisions on the acceptance of the peer-reviewed manuscripts.
Preprints
SSRR considers publication of manuscripts that contain information of primary research previously posted on a recognized non-profit preprint server. However, authors are not allowed to submit their paper to any preprint servers after it has been submitted to SSRR.
Authors must acknowledge, during submission, preprint server deposition with a link to your published manuscript and provide the associated accession number or DOI. No revisions must be posted to the preprint server after the manuscript submission to SSRR. If the manuscript is accepted for publication in SSRR, the authors must indicate on the preprint server that the final peer-reviewed version of the article is published in SSRR and are responsible for updating the archived pre-print with a DOI and link to the published version of the article in SSRR.
Confidentiality
All manuscript details, author information, reviewer identities, comments to the editors and the authors, and the content of the decision letter are considered privileged information and will never be disclosed to third parties.
Authorship/Contributorship
All authors listed in the manuscript must meet the following criteria of contribution described by the ICMJE in the Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly work in Medical Journals.
- Substantial contributions to the conception or design of the research or the acquisition and analysis of data, and
- Drafting the work or it critically for important intellectual content, and
- Final approval of the version to be published, and
- Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
The corresponding author must ensure that a manuscript is read and approved by all authors prior to submission.
Contributors who do not meet all 4 criteria for authorship above should not be listed as authors. Guest or honorary authorship is not permitted.
Those who do not qualify for authorship may be acknowledged individually or together as a group under the single heading, “Acknowledgements,” on the title page. Examples of activities that do not qualify a contributor for authorship are acquisition of funding, general supervision of a research group, or general administrative support and writing assistance, technical editing, language editing, and proofreading.
Authors should discuss, determine and (if they exist) settle any disagreements about the order of authorship before submitting their manuscript. Final author order must be established by the end of the revision phase of the peer review process. Any changes such as order, addition, and deletion of authors, between the initial manuscript submission and the final decision, should be discussed and approved by all authors. Any request for such changes must be explained in the Change of Authorship Form, which must be signed by all authors.
Adding, deleting, or changing the author names and their order is not permitted after the acceptance of the manuscript for publication.
Use of Artificial Intelligence (AI)-Assisted Tools/Technologies
In consonance with the COPE’s position statement, WAME’s recommendations, and ICMJE’s Recommendation, SSRR does not allow artificial intelligence (AI)-assisted tools/technologies such as Large Language Models (LLMs), chatbots, or image creators to be listed as author or co-author. As described in the ICMJE, those tools cannot be responsible for the accuracy, integrity, and originality of the work, thus they do not meet the ICMJE’s criteria for authorship listed above. The authors (humans) are fully responsible for any materials of the submitted work, including the use of AI-assisted tools or technologies. AI should not be cited as authors. Authors (humans) are also responsible for plagiarism including the in text and AI-produced images. Authors must disclose, upon submission and in the Materials and Methods (or similar section), any use of AI-assisted tools or technologies in the writing of a manuscript, production of images or graphical elements of the paper, or in the collection and analysis of data.
Conflict of Interest and Sources of Funding
Authors must explicitly state whether potential conflicts of interest (COI) exist or not. This includes, but is not limited to, agreements for research support (including research funding and provision of equipment or materials), honoraria (such as lecture fees), consulting, employment, promotional fees, advisory role, stock ownership, patent/licensing fees, and any other financial, institutional or personal relationships with biotechnology manufacturers, pharmaceutical companies, or other commercial organizations that have any interest in the subject matter, materials, or process(es) discussed in the manuscript. Any possible COI related to the study presented in the manuscript must be disclosed on the title page under the heading “Conflicts of Interest” using the following examples for each author:
“A (author name) received honoraria from Z (entity name); B holds an advisory role in Y; C is an employee of Company X.”
If a manuscript is accepted for publication, the disclosures will be published as they appear in this section. If there are no COIs, the authors should state “The authors declare that there are no relevant conflicts of interest” on the title page.
All authors will receive e-mail notification to confirm and complete their COI disclosure forms (e-forms) after manuscript submission.
All sources of funding from entities such as governmental or non-profit organizations, that are relevant to the study, should be acknowledged on the title page under the heading “Sources of Funding.” You must ensure that the full, correct details of your funder(s) and any relevant grant numbers are included.
Research Ethics
- Clinical research included in articles, which report on human subjects or materials of human origin, must comply with the provisions of the Declaration of Helsinki, and it must be mentioned that the study has been approved by the relevant institutional or national review board (IRB). If no approval from any IRB was required, that must be explicitly stated in the manuscript. Those researchers who do not have institutional or national ethics review committees should follow the principles outlined in the Declaration of Helsinki.
- Any studies involving human subjects must clearly indicate that written consent has been obtained from all patients and relevant persons (such as the parent or legal guardian) to publish the information, including photographs.
- Any data or information such as patient names, initials, hospital patient identification codes (patient IDs), specific dates, or any other information that may identify patients must not be presented anywhere in the manuscript, including the Figures and Tables. All pictures should focus on the affected areas only.
- Articles reporting on data from animal testing must indicate in the “Subjects and Methods” section the approval of the testing design by the affiliated institution’s Animal Care and Use Committee, without mentioning the name of the institution, instead using the phrase “our affiliated institution.”
- Authors of articles reporting on new DNA sequences must furnish those data to the GenBank and include the accession number in the article.
Medical Devices and Drugs
SSRR does NOT accept a manuscript that includes medical device(s) or drug(s), which are
- Not approved by the government or public agencies of the author’s country.
- Not intended for clinical use for humans.
- Prohibited by the local regulations of the author’s country.
- Not properly used in accordance with the local regulations of the author’s country.
If the device(s)/drug(s) that is/are the subject of, or used in, the manuscript is/are not approved officially for its(their) clinical use for humans or were used experimentally by the author, the author must provide the following information in the title page of the manuscript; 1) name of the device(s)/drug(s), 2) name of the author’s country, and 3) the regulations or conditions regarding the device(s)/drug(s) in the author’s country.
If the device(s)/drug(s) that is/are the subject of, or used in, the manuscript is/are exempt from the regulations of author’s country, the author must state reason in the title page of the manuscript.
Ethical Policies and Handling of Misconduct
The Editorial Committee of SSRR follows the recommended procedures outlined by COPE International Standards for responsible research publication for authors and editors when dealing with allegations of misconduct including complaints about publication ethics, scientific content, and peer-review process during submission or after acceptance/publication of the manuscripts. Please see the journal’s Ethical Policies for the details.
Proofing and Revision after Acceptance
After the acceptance of a manuscript for publication, galley proofs will be available to the authors for corrections of minor errors such as spelling errors and omitted characters or letters. Any other corrections and revisions after the acceptance of a manuscript are not permitted unless requested by the Editorial Committee of the Journal. Authors are expected to perform the proofing, as instructed by the Editorial Office. Upon completion of the proofing, authors should immediately e-mail the revised proof to the publisher.
Article Processing Charge (APC)
Submission and publication of all types of papers in SSRR is FREE OF CHARGE.
Copyright
Copyright of articles and their contents published in SSRR belong to the Japanese Society for Spine Surgery and Related Research. SSRR is an Open Access journal distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (https://creativecommons.org/licenses/by-nc-nd/4.0/). Anyone may download, reuse, copy, reprint, or distribute articles published in the journal for not-for-profit purposes if they cite the original authors and source properly. If you remix, transform, or build upon the material, you may not distribute the modified material. For for-profit or commercial use, written permission by the Editorial Committee of SSRR is mandatory. The license can be found on the last page of the published PDF of the article.
[Print version] Instructions for Authors




